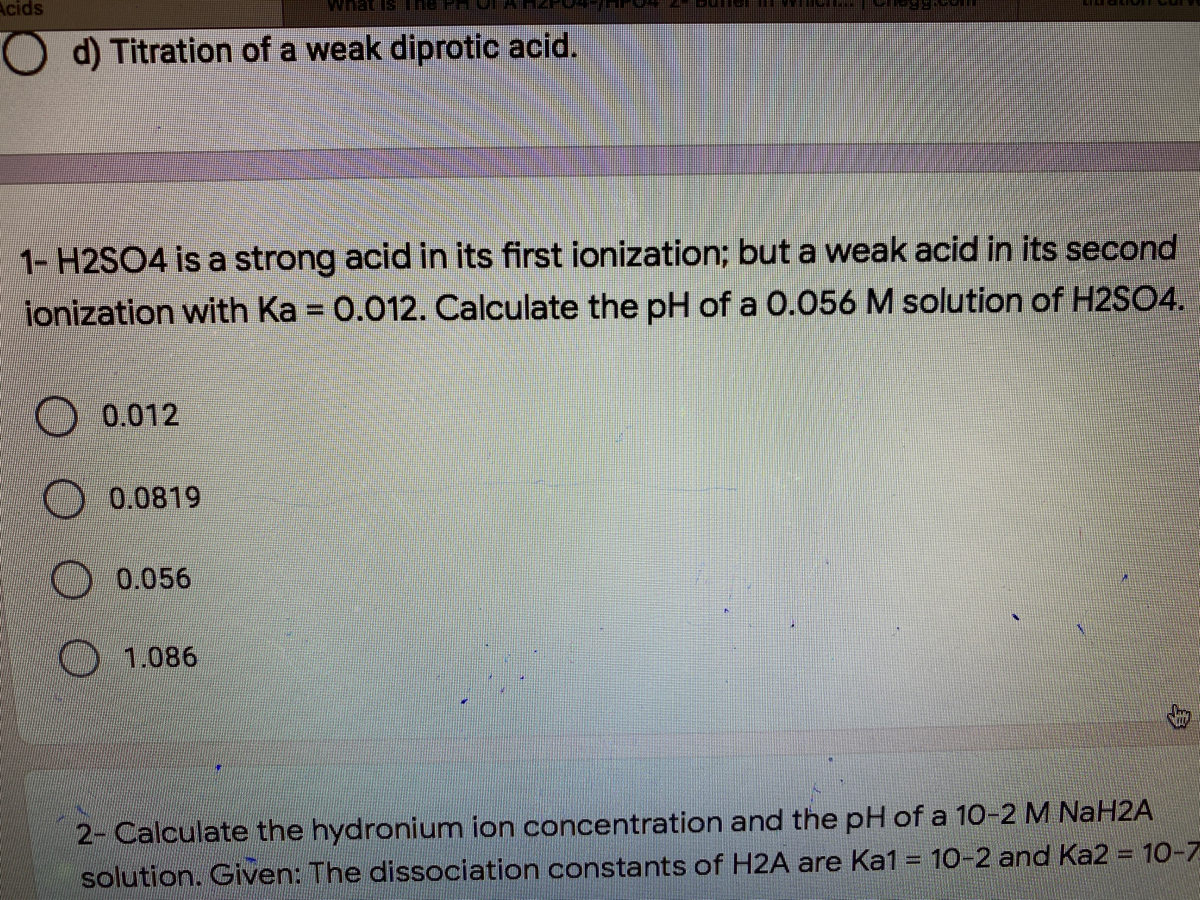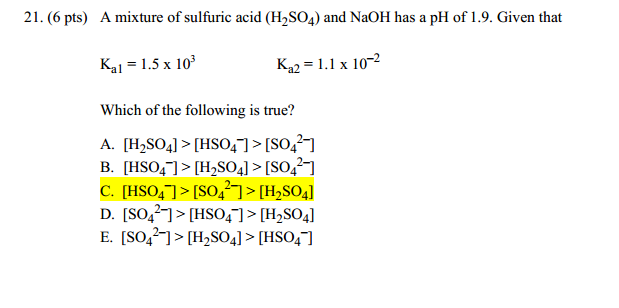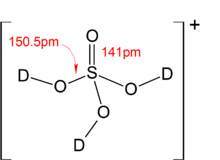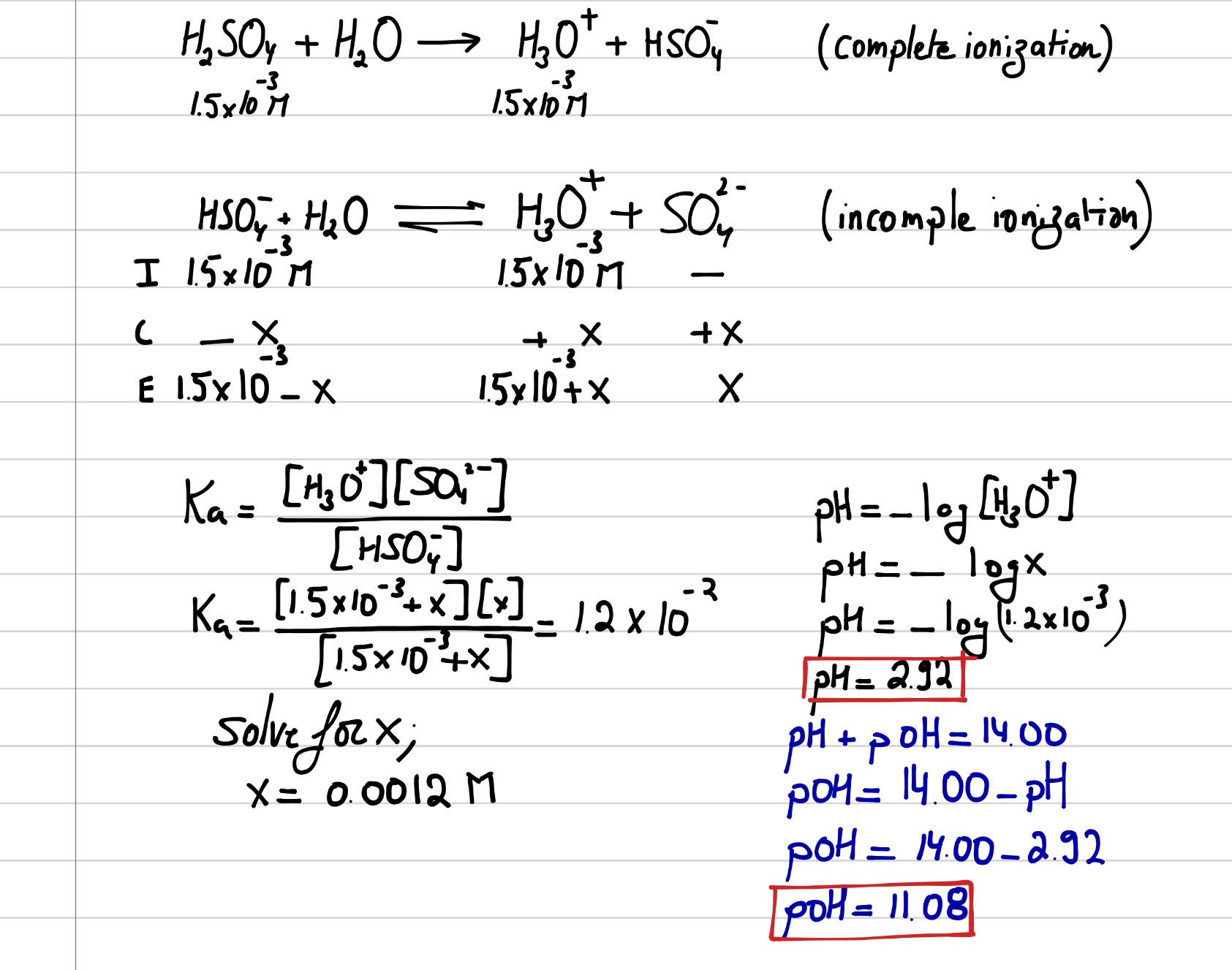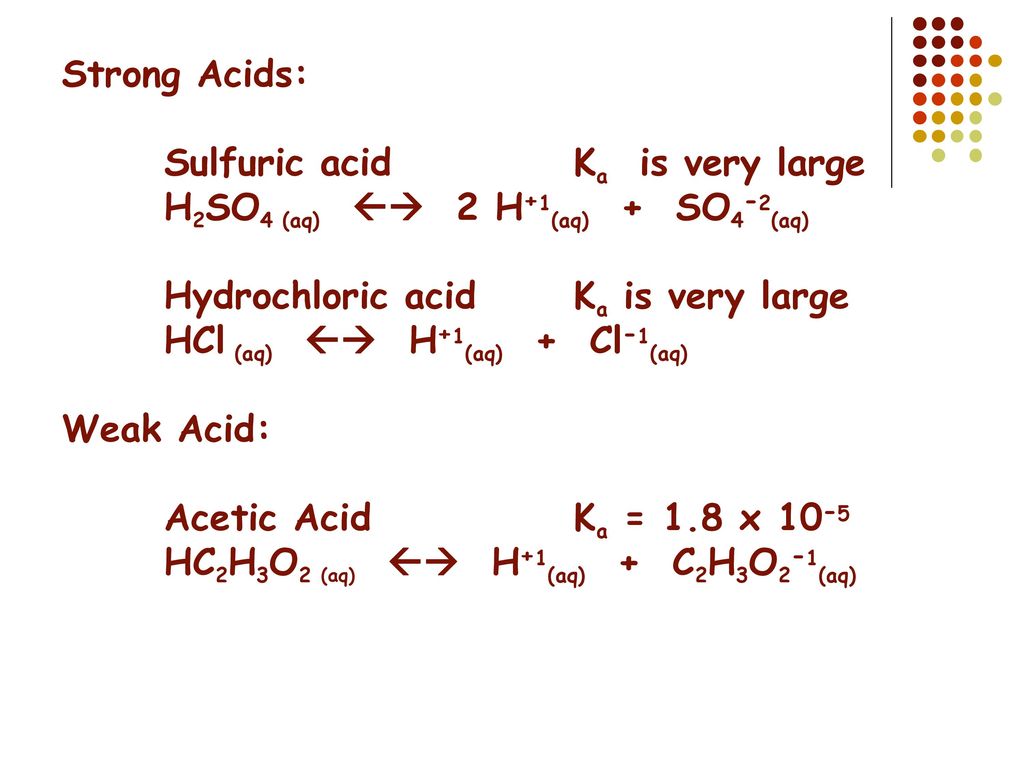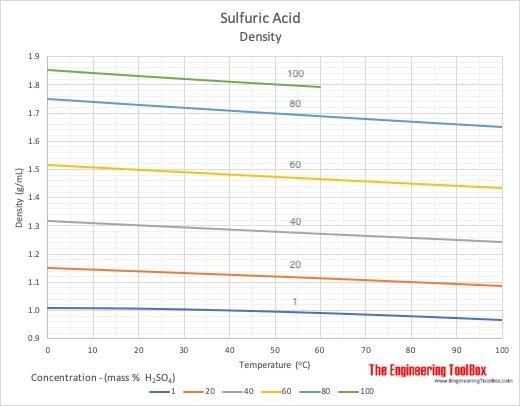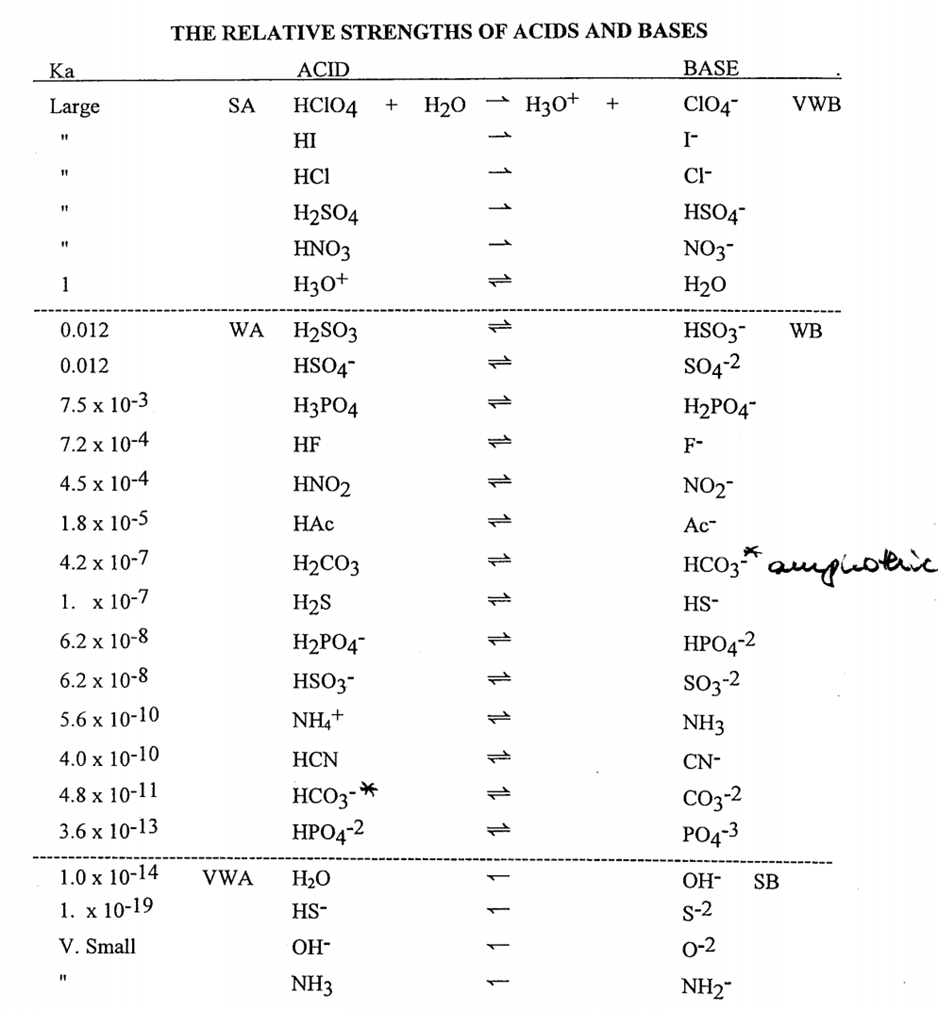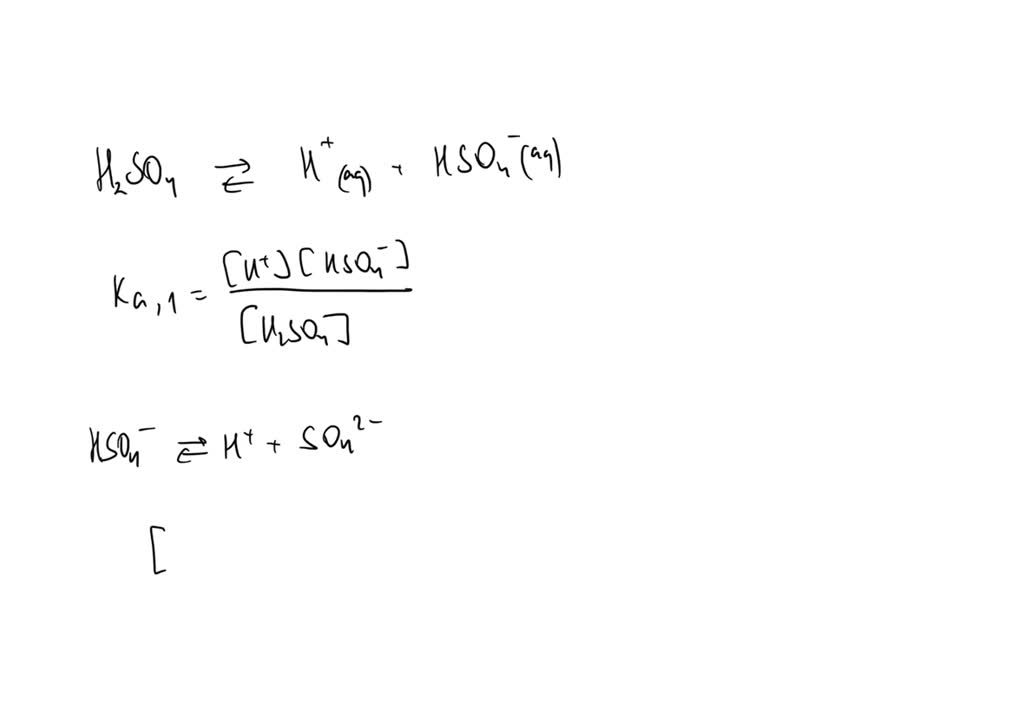
SOLVED: Write the acid dissociation equilibrium for H2SO4 in water and write the Ka expression. Is this a strong or weak acid? Are products, reactants, or both favored at equilibrium? Answer: This
![PDF] Diffusion Coefficient and Solubility of Isobutene and trans-2-Butene in Aqueous Sulfuric Acid Solutions | Semantic Scholar PDF] Diffusion Coefficient and Solubility of Isobutene and trans-2-Butene in Aqueous Sulfuric Acid Solutions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5a60269de402e1d6f7763a1a9180b124114453aa/5-Table4-1.png)
PDF] Diffusion Coefficient and Solubility of Isobutene and trans-2-Butene in Aqueous Sulfuric Acid Solutions | Semantic Scholar

Kinetic parameters for HER in 0.5 M H2SO4 solution in the presence of... | Download Scientific Diagram

Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A

Sulfuric Acid H2so4 Ballandstick Model Molecular And Chemical Formula Stock Illustration - Download Image Now - iStock

Question 26 of 32What is the equation for the acid dissociation constant, Ka, of carbonic acid? - brainly.com

If Ka1 and Ka2 of sulphuric acid are 1 × 10^-2 and 1 × 10^-6 respectively, then concentration of sulphate ions in 0.01 MH2SO4 solution will be: