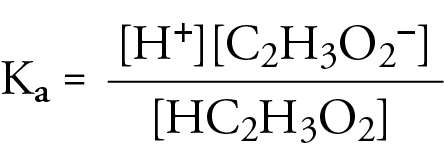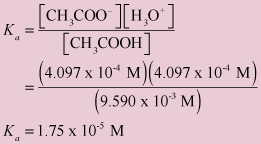What is the pH of a 10 mM solution of acetic acid (CH3COOH)? Acetic Acid Ka= 1.76 x 10^{-5} M. | Homework.Study.com
How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora
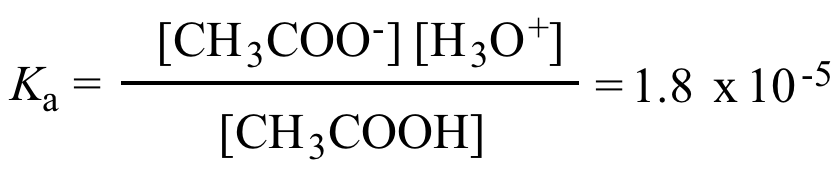
Illustrated Glossary of Organic Chemistry - Acid ionization constant (acid dissociation constant; Ka)
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://i.ytimg.com/vi/AufT6_CoFWY/maxresdefault.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
✓ Solved: Ka for acetic acid is 1.7× 10^-5 at 25°C. A buffer solution is made by mixing 52.1 mL of 0.122...
THE DISSOCIATION CONSTANT OF ACETIC ACID FROM 0 TO 35° CENTIGRADE1 | Journal of the American Chemical Society
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora

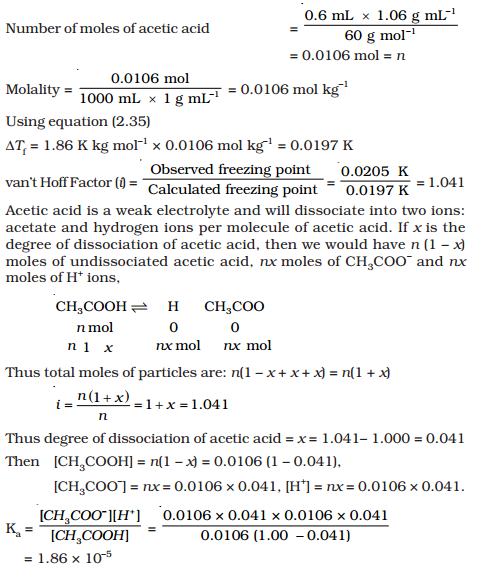
A student prepared 0.10M acetic acid solution and experimentally measured its pH to be 2.88. Calculate Ka - Brainly.in